In this post, we are going to try a CITE-seq normalization method called dsb published in
Normalizing and denoising protein expression data from droplet-based single cell profiling
two major components of protein expression noise in droplet-based single cell experiments: (1) protein-specific noise originating from ambient, unbound antibody encapsulated in droplets that can be accurately estimated via the level of “ambient” ADT counts in empty droplets,
and (2) droplet/cell-specific noise revealed via the shared variance component associated with isotype antibody controls and background protein counts in each cell. We develop an R software package, “dsb” (denoised and scaled by background), the first dedicated low-level normalization method developed for protein ADT data, to correct for both of these noise sources without experimental modifications
Another interesting blog post from the same author: CD4 protein vs CD4 mRNA in CITE-seq data
This is Part 4 of a series of posts on CITE-seq data analysis. You can find the previous posts:
- Part 1 How to use Salmon/Alevin to preprocess CITE-seq data
- Part 2 CITE-seq downstream analysis: From Alevin output to Seurat visualization
- Part 3 Centered log ratio (CLR) normalization for CITE-seq protein count data
To follow the tutorial, you can download the associated data from here.
library(here)
library(dplyr)
suppressPackageStartupMessages({
library(fishpond)
library(ggplot2)
library(SingleCellExperiment)
library(Seurat)
library(DropletUtils)
})
# set the seed
set.seed(123)
#gex_q <- loadFry(here('data/NLS088_OV/CITE-seq/alevin_rna/af_quant'))
#fb_q <- loadFry(here('data/NLS088_OV/CITE-seq/alevin_adt/af_quant'))
# I saved the above objs first to rds files, now just read them back
fb_q<- readRDS("~/blog_data/CITEseq/fb_q.rds")
gex_q<-readRDS("~/blog_data/CITEseq/gex_q.rds")
gex_q<- counts(gex_q)
fb_q<- counts(fb_q)
# only 244 barcode are the same, not right
length(intersect(colnames(gex_q), colnames(fb_q)))#> [1] 244translate barcode
If the feature barcoding protocol use TotalSeq B or C, as mentioned by 10x Genomics, the cellular barcodes of RNA and the feature barcode library would not be exactly the same. To match the cellular barcodes across technologies the cellular barcodes needs to be transformed based on a mapping file. The code to perform the mapping is here and more information from 10x Genomic is at https://kb.10xgenomics.com/hc/en-us/articles/360031133451-Why-is-there-a-discrepancy-in-the-3M-february-2018-txt-barcode-whitelist-.
library(readr)
# the mapping file
map <- read_tsv('~/blog_data/CITEseq/3M-february-2018.txt.gz', col_names = FALSE)
head(map)#> # A tibble: 6 × 2
#> X1 X2
#> <chr> <chr>
#> 1 AAACCCAAGAAACACT AAACCCATCAAACACT
#> 2 AAACCCAAGAAACCAT AAACCCATCAAACCAT
#> 3 AAACCCAAGAAACCCA AAACCCATCAAACCCA
#> 4 AAACCCAAGAAACCCG AAACCCATCAAACCCG
#> 5 AAACCCAAGAAACCTG AAACCCATCAAACCTG
#> 6 AAACCCAAGAAACGAA AAACCCATCAAACGAArnaToADT <- map$X1
names(rnaToADT) <- map$X2
colnames(gex_q) <- rnaToADT[colnames(gex_q)]
dim(gex_q)#> [1] 36601 83480dim(fb_q)#> [1] 17 64016length(intersect(colnames(gex_q), colnames(fb_q)))#> [1] 60985# 60985
common_barcode<- intersect(colnames(gex_q), colnames(fb_q))subset only the common cell barcodes for the protein and RNA.
gex_q<- gex_q[, common_barcode]
fb_q<- fb_q[, common_barcode]
dim(gex_q)#> [1] 36601 60985dim(fb_q)#> [1] 17 60985identify empty cells
use DropletUtils to remove empty cells
e.out <- emptyDrops(gex_q)
is.cell<- e.out$FDR <= 0.01
is.cell[is.na(is.cell)]<- FALSE
gex_q.filt<- gex_q[, is.cell]
dim(gex_q.filt)#> [1] 36601 713Knee plot
tot_counts <- colSums(gex_q)
df <- tibble(total = tot_counts,
rank = row_number(dplyr::desc(total))) %>%
distinct() %>%
dplyr::arrange(rank)
ggplot(df, aes(rank, total)) +
geom_path() +
scale_x_log10() +
scale_y_log10() +
annotation_logticks() +
labs(x = "Barcode rank", y = "Total UMI count") +
geom_vline(xintercept = 720, linetype = 2, color = "red") +
annotate("text", x=720, y=1000, label="720 cells", angle= 0, size= 5, color="blue")+
theme_bw(base_size = 14)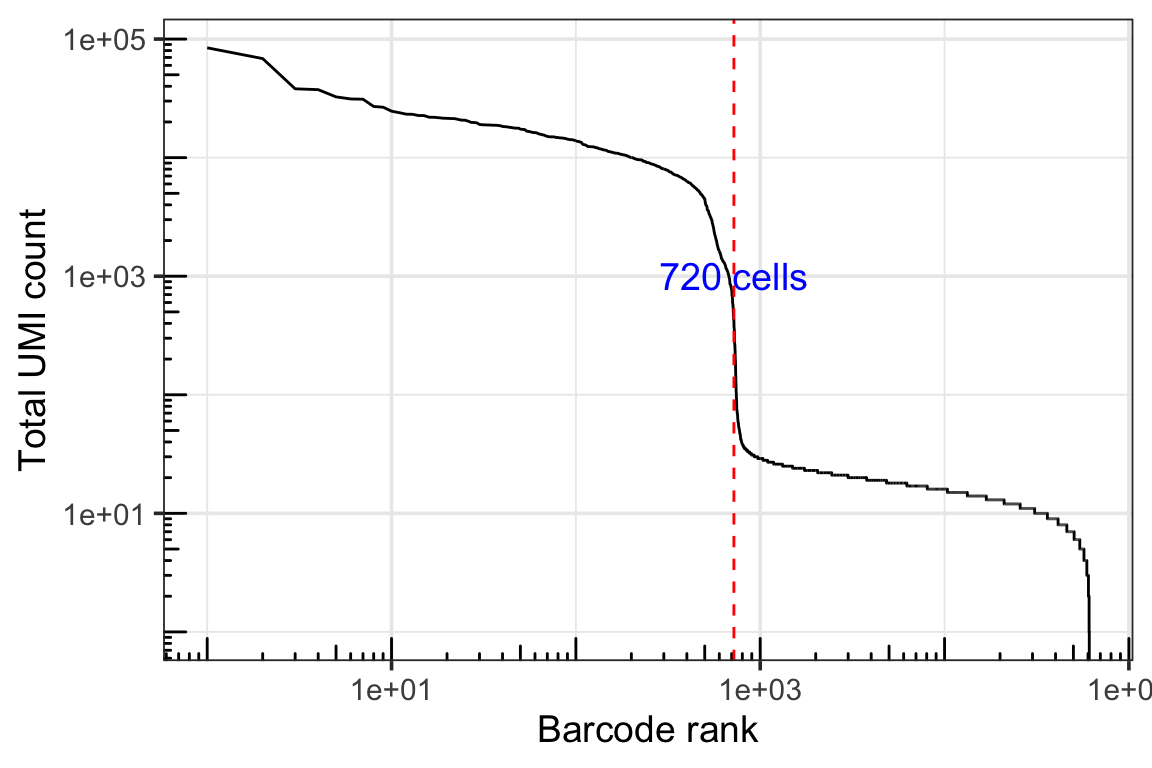
use the empty droplet as controls for ADT background removing
stained_cells<- colnames(gex_q.filt)
background<- setdiff(colnames(gex_q), stained_cells)
length(stained_cells)#> [1] 713length(background)#> [1] 60272convert ENSEMBLE ID to gene symobol for the gene expression matrix
There is one more problem. the ids in the gene expression matrix are ENSEBML IDs. Let’s convert ENSEMBLE ID to gene symbol.
#convert ENSEMBLE ID to gene symobol
gex_q.filt[1:5, 1:5]#> 5 x 5 sparse Matrix of class "dgCMatrix"
#> CCGAACGTCAAGTATC ACTCCCAAGCGACAGC GCATCTCCACACTGAT
#> ENSG00000235146 . . .
#> ENSG00000237491 . . .
#> ENSG00000228794 . . .
#> ENSG00000272438 . . .
#> ENSG00000230699 . . .
#> CTCAGAATCGTAGATT GCCATTCAGAATCGGT
#> ENSG00000235146 . .
#> ENSG00000237491 . 2
#> ENSG00000228794 . .
#> ENSG00000272438 . .
#> ENSG00000230699 . .gene_dat<- rownames(gex_q.filt) %>%
tibble::enframe(value = "gene_id")
# read in the gtf file
gtf <- rtracklayer::import('~/blog_data/CITEseq/genes.gtf')
gtf_df<- as.data.frame(gtf)
filter_gene_names <- gtf_df %>%
dplyr::filter(type == "gene") %>%
dplyr::select(gene_id, gene_name) #%>% some version of gtf has version number in the ENSEMBL id, you can
# remove it
#mutate(gene_id = stringr::str_replace(gene_id, "(ENSG.+)\\.[0-9]+", "\\1"))
gene_dat <- left_join(gene_dat, filter_gene_names)
# there are some duplicated
head(gene_dat)#> # A tibble: 6 × 3
#> name gene_id gene_name
#> <int> <chr> <chr>
#> 1 1 ENSG00000235146 AC114498.1
#> 2 2 ENSG00000237491 LINC01409
#> 3 3 ENSG00000228794 LINC01128
#> 4 4 ENSG00000272438 AL645608.6
#> 5 5 ENSG00000230699 AL645608.2
#> 6 6 ENSG00000187961 KLHL17all.equal(rownames(gex_q.filt), gene_dat$gene_id)#> [1] TRUEall.equal(rownames(gex_q), gene_dat$gene_id)#> [1] TRUE# make.name will convert MT- to MT.
rownames(gex_q)<- make.names(gene_dat$gene_name, unique=TRUE)
rownames(gex_q.filt)<- make.names(gene_dat$gene_name, unique=TRUE)
gex_q.filt[1:5, 1:5]#> 5 x 5 sparse Matrix of class "dgCMatrix"
#> CCGAACGTCAAGTATC ACTCCCAAGCGACAGC GCATCTCCACACTGAT CTCAGAATCGTAGATT
#> AC114498.1 . . . .
#> LINC01409 . . . .
#> LINC01128 . . . .
#> AL645608.6 . . . .
#> AL645608.2 . . . .
#> GCCATTCAGAATCGGT
#> AC114498.1 .
#> LINC01409 2
#> LINC01128 .
#> AL645608.6 .
#> AL645608.2 .Quality control cells and background droplets
Follow https://cran.r-project.org/web/packages/dsb/vignettes/end_to_end_workflow.html for more quality control
# create metadata of droplet QC stats used in standard scRNAseq processing
mtgene<- grep(pattern = "^MT\\.", rownames(gex_q), value = TRUE) # used below
md<- data.frame(
rna.size = log10(Matrix::colSums(gex_q)),
prot.size = log10(Matrix::colSums(fb_q)),
n.gene = Matrix::colSums(gex_q > 0),
mt.prop = Matrix::colSums(gex_q[mtgene, ]) / Matrix::colSums(gex_q)
)
# add indicator for barcodes Cell Ranger called as cells
md$drop.class<- ifelse(rownames(md) %in% stained_cells, 'cell', 'background')
# remove barcodes with no evidence of capture in the experiment, this is log scale
# so > 1 count
md<- md[md$rna.size > 0 & md$prot.size > 0, ]# install.packages("ggside")
library(ggside)
ggplot(md, aes(x= prot.size)) +
geom_histogram(aes(fill = drop.class), bins = 50, color = "black") +
ggside::geom_xsidedensity(aes(y = after_stat(density), color = drop.class, fill = drop.class),
position = "stack", alpha = 0.4) +
scale_xsidey_continuous(minor_breaks = NULL)+
theme_bw() 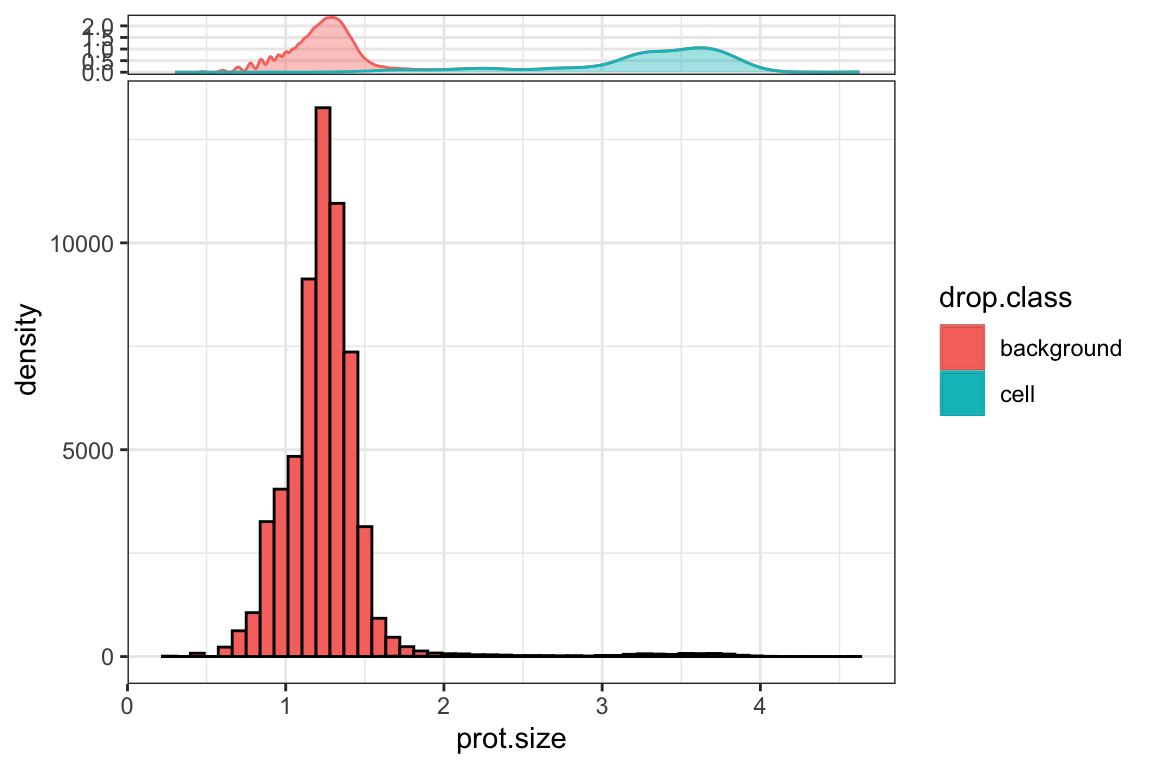
Quality control for the background cells:
prot.size is the log10 of total number of ADT counts in each cell.
ggplot(md, aes(x = log10(n.gene), y = prot.size )) +
theme_bw() +
geom_bin2d(bins = 100) +
scale_fill_viridis_c(option = "C") +
facet_wrap(~drop.class) +
geom_hline(yintercept = 2, color = "red", linetype = 2) +
geom_vline(xintercept = 2, color = "red", linetype = 2)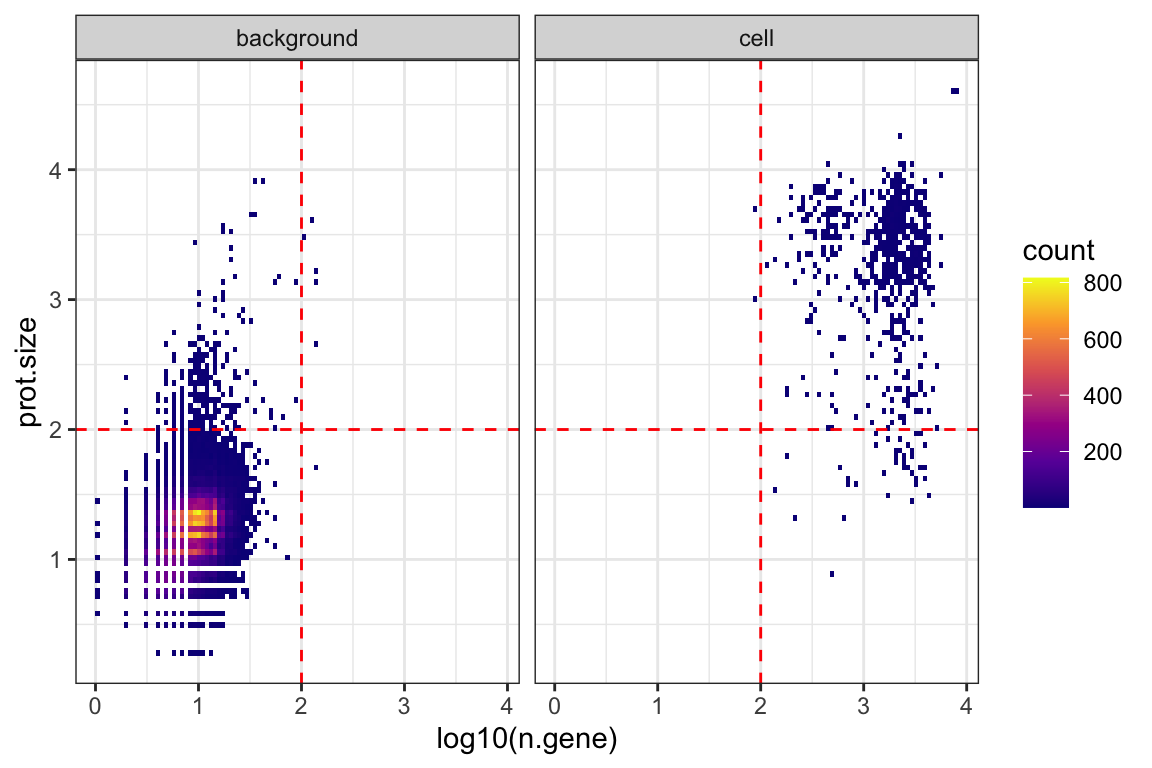
subset the high-quality empty droplets
background_drops<- rownames(
md[ md$prot.size > 0.5 &
md$prot.size < 2 &
md$rna.size < 2, ]
)
background.adt.mtx = as.matrix(fb_q[ , background_drops])
dim(background.adt.mtx)#> [1] 17 59732~60,000 empty droplets are included after QC. Normalized values are robust to background thresholds used, so long as one does not omit the major background population.
filter the adt count matrix
fb_q.filt<- fb_q[, stained_cells]
dim(gex_q.filt)#> [1] 36601 713dim(fb_q.filt)#> [1] 17 713fb_q.filt[1:5, 1:5]#> 5 x 5 sparse Matrix of class "dgCMatrix"
#> CCGAACGTCAAGTATC ACTCCCAAGCGACAGC GCATCTCCACACTGAT CTCAGAATCGTAGATT
#> CD3 4 2 8 10
#> CD4 483 192 185 316
#> CD8a 16 9 3 9
#> CD14 1148 662 13 850
#> CD15 36 32 17 38
#> GCCATTCAGAATCGGT
#> CD3 3
#> CD4 20
#> CD8a 1
#> CD14 73
#> CD15 3Normalize protein data with the DSBNormalizeProtein Function
# install.packages("dsb")
library(dsb)
rownames(fb_q.filt)#> [1] "CD3" "CD4" "CD8a" "CD14" "CD15" "CD16" "CD56" "CD19"
#> [9] "CD25" "CD45RA" "CD45RO" "PD-1" "TIGIT" "CD127" "IgG2a" "IgG1"
#> [17] "IgG2b"# define isotype controls
isotype.controls<- c("IgG2a", "IgG1",
"IgG2b")
# normalize and denoise with dsb with
cells.dsb.norm<- DSBNormalizeProtein(
cell_protein_matrix = fb_q.filt,
empty_drop_matrix = background.adt.mtx,
denoise.counts = TRUE,
use.isotype.control = TRUE,
isotype.control.name.vec = isotype.controls)#> [1] "correcting ambient protein background noise"
#> [1] "some proteins with low background variance detected check raw and normalized distributions. protein stats can be returned with return.stats = TRUE"
#> [1] "IgG2b"
#> [1] "fitting models to each cell for dsb technical component and removing cell to cell technical noise"The function returns a matrix of normalized protein values which can be integrated with Seurat.
cells.dsb.norm[1:5, 1:5]#> CCGAACGTCAAGTATC ACTCCCAAGCGACAGC GCATCTCCACACTGAT CTCAGAATCGTAGATT
#> CD3 0.4066237 -0.3896268 -0.1050716 5.067701
#> CD4 26.1671731 19.9957852 17.6891651 25.105814
#> CD8a 6.9744573 4.3026643 -2.7203584 7.086775
#> CD14 34.3506644 30.3298611 2.7421026 33.577690
#> CD15 8.1007843 7.6738079 2.7043322 10.192798
#> GCCATTCAGAATCGGT
#> CD3 4.713502
#> CD4 10.199216
#> CD8a 5.370481
#> CD14 17.313187
#> CD15 3.314175analyze in Seurat
pbmc <- CreateSeuratObject(counts = gex_q.filt)
# add dsb normalized matrix "cell.adt.dsb" to the "ADT" data (not counts!) slot
adt_assay <- CreateAssayObject(data = cells.dsb.norm)
#adt_assay <- CreateAssayObject(counts = fb_q.filt)
# add this assay to the previously created Seurat object
pbmc[["ADT"]] <- adt_assay
library(RColorBrewer)
my_cols = brewer.pal(8,"Dark2")
DefaultAssay(pbmc) <- "RNA"
pbmc[["percent.mt"]] <- PercentageFeatureSet(pbmc, pattern = "^MT.")
VlnPlot(pbmc, features = c("nFeature_RNA", "nCount_RNA", "percent.mt"), ncol = 3)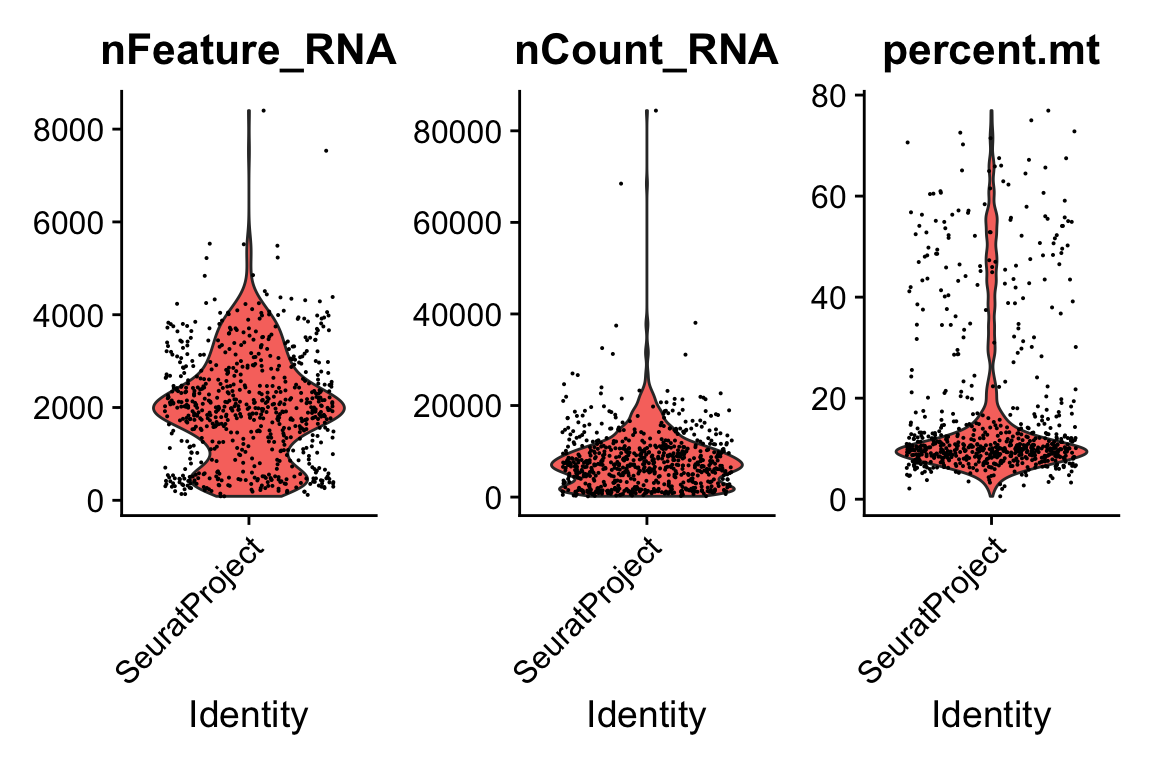
pbmc <- subset(pbmc, subset = nFeature_RNA > 200 & nFeature_RNA < 4500 & percent.mt < 20)Routine processing for the RNA data.
pbmc <- NormalizeData(pbmc)
pbmc <- FindVariableFeatures(pbmc)
pbmc <- ScaleData(pbmc)
pbmc <- RunPCA(pbmc, verbose = FALSE)
pbmc <- FindNeighbors(pbmc, dims = 1:30)
pbmc <- FindClusters(pbmc, resolution = 0.6, verbose = FALSE)
pbmc <- RunUMAP(pbmc, dims = 1:30)
DimPlot(pbmc, label = TRUE,cols=alpha(my_cols,0.66))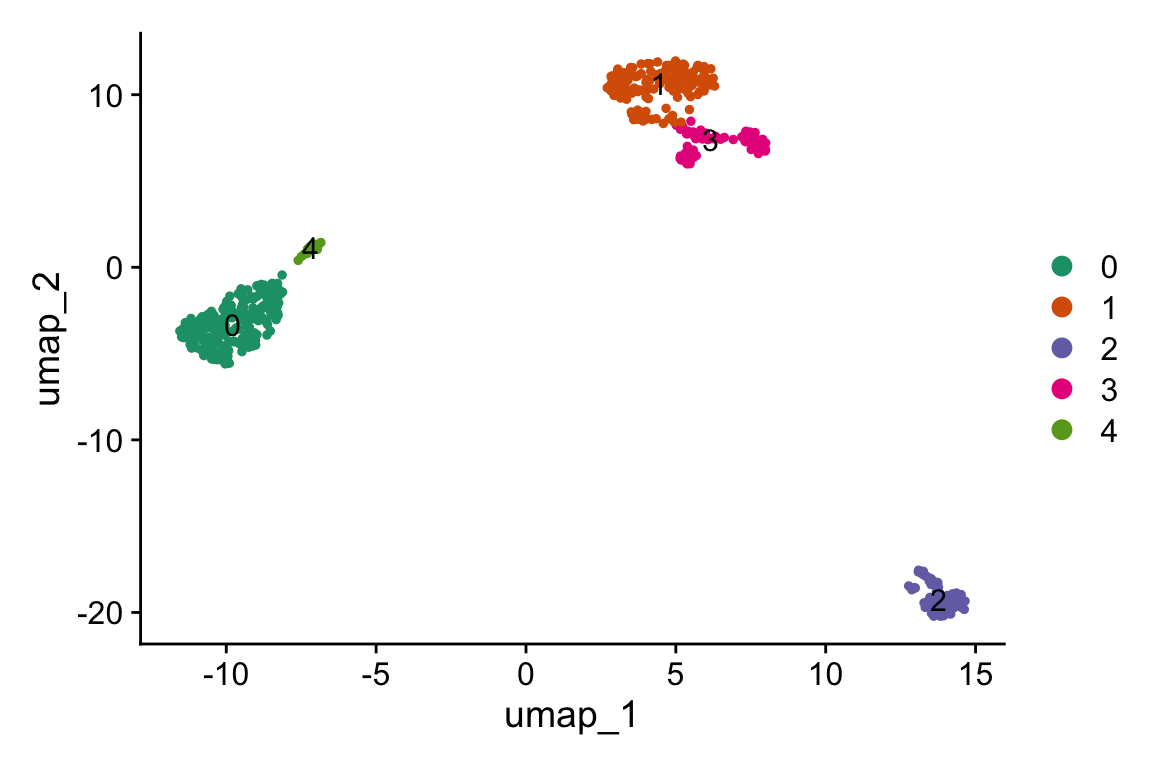
marker genes
markers<- presto::wilcoxauc(pbmc)
presto::top_markers(markers, n = 20)#> # A tibble: 20 × 6
#> rank `0` `1` `2` `3` `4`
#> <int> <chr> <chr> <chr> <chr> <chr>
#> 1 1 LYZ BCL11B CD74 NKG7 LST1
#> 2 2 S100A9 LDHB CD79A CTSW CDKN1C
#> 3 3 S100A8 RPS27A MS4A1 GZMA FCGR3A
#> 4 4 VCAN RPS12 CD37 CST7 AIF1
#> 5 5 FCN1 RPS18 IGHM GZMM SMIM25
#> 6 6 MNDA RPS15A HLA.DPB1 HCST HES4
#> 7 7 S100A12 IL7R CD79B PRF1 MS4A7
#> 8 8 CTSS RPL36 BANK1 CCL5 TCF7L2
#> 9 9 S100A6 RPS29 IGHD MATK FCER1G
#> 10 10 FGL2 TPT1 HLA.DQA1 KLRB1 SIGLEC10
#> 11 11 CD14 RPS6 HLA.DRB1 CD7 RHOC
#> 12 12 MS4A6A RPS3 HLA.DRA KLRG1 CSF1R
#> 13 13 KLF4 RPS25 RALGPS2 HOPX SAT1
#> 14 14 GRN EEF1A1 HLA.DPA1 CD247 LYN
#> 15 15 AC020656.1 RPS16 IGKC B2M COTL1
#> 16 16 MPEG1 RPS3A HLA.DQB1 ARL4C VASP
#> 17 17 APLP2 RPL10 LINC00926 KLRD1 RRAS
#> 18 18 SRGN CD3D TNFRSF13C HLA.C NAP1L1
#> 19 19 TSPO RPL32 HVCN1 CALM1 TMSB4X
#> 20 20 CEBPD RPL34 BCL11A KLRK1 BID- cluster 0 is CD14 monocytes
- cluster 4 is CD16 monocytes
- cluster 1 is T cells
- cluster 3 is NK cells
- cluster 2 is B cells
clustered dot plot
celltypes<- pbmc@meta.data %>%
mutate(annotation = case_when(
seurat_clusters == 0 ~ "CD14 mono",
seurat_clusters == 4 ~ "CD16 mono",
seurat_clusters == 1 ~ "T cells",
seurat_clusters == 3 ~ "NK cells",
seurat_clusters == 2 ~ "B cells")) %>%
pull(annotation)
pbmc$celltypes<- celltypes
genes_to_plot<- c("CD14", "FCGR3A", "SIGLEC10", "FCER1G", "CD3D", "CD4", "CD8A", "NKG7", "KLRB1", "CD79A", "MS4A1")
scCustomize::Clustered_DotPlot(pbmc, features = genes_to_plot,
group.by="celltypes",
plot_km_elbow = FALSE)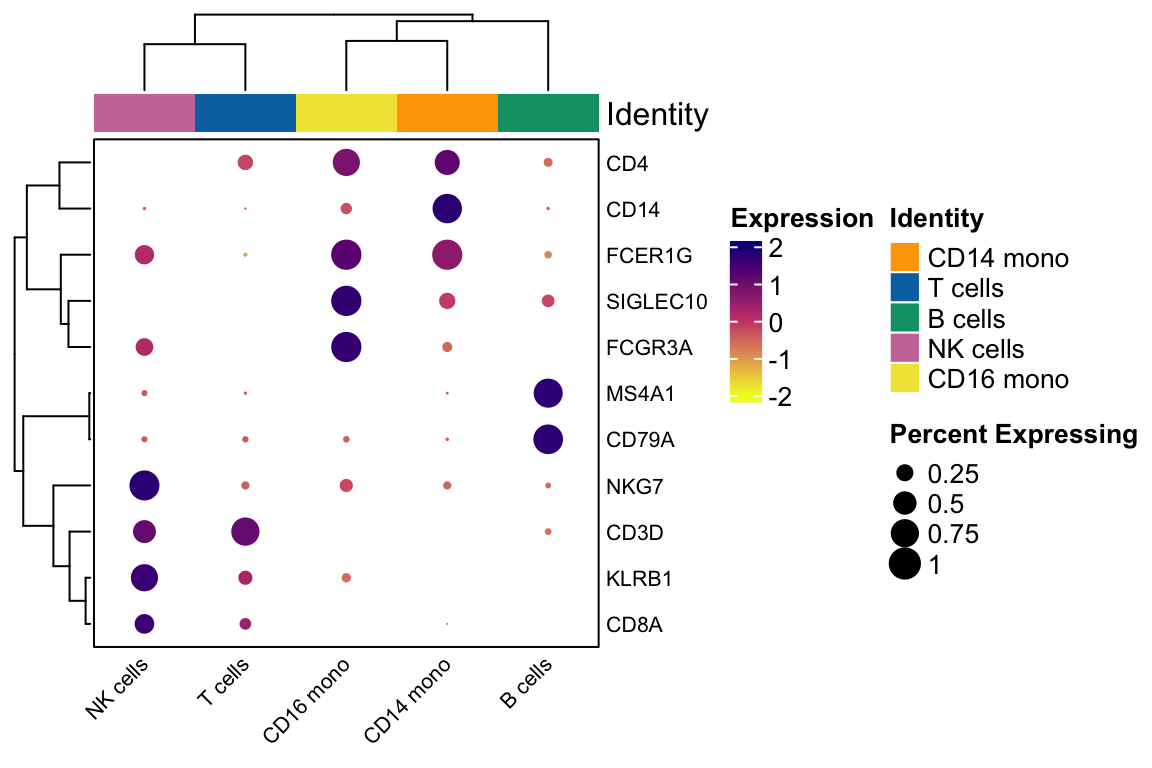
DefaultAssay(pbmc) <- "ADT"
p1 <- FeaturePlot(pbmc, "CD19", cols = c("lightgrey", "darkgreen")) + ggtitle("CD19 protein")
DefaultAssay(pbmc) <- "RNA"
p2 <- FeaturePlot(pbmc, "CD19", order = TRUE) + ggtitle("CD19 RNA")
# place plots side-by-side
p1 | p2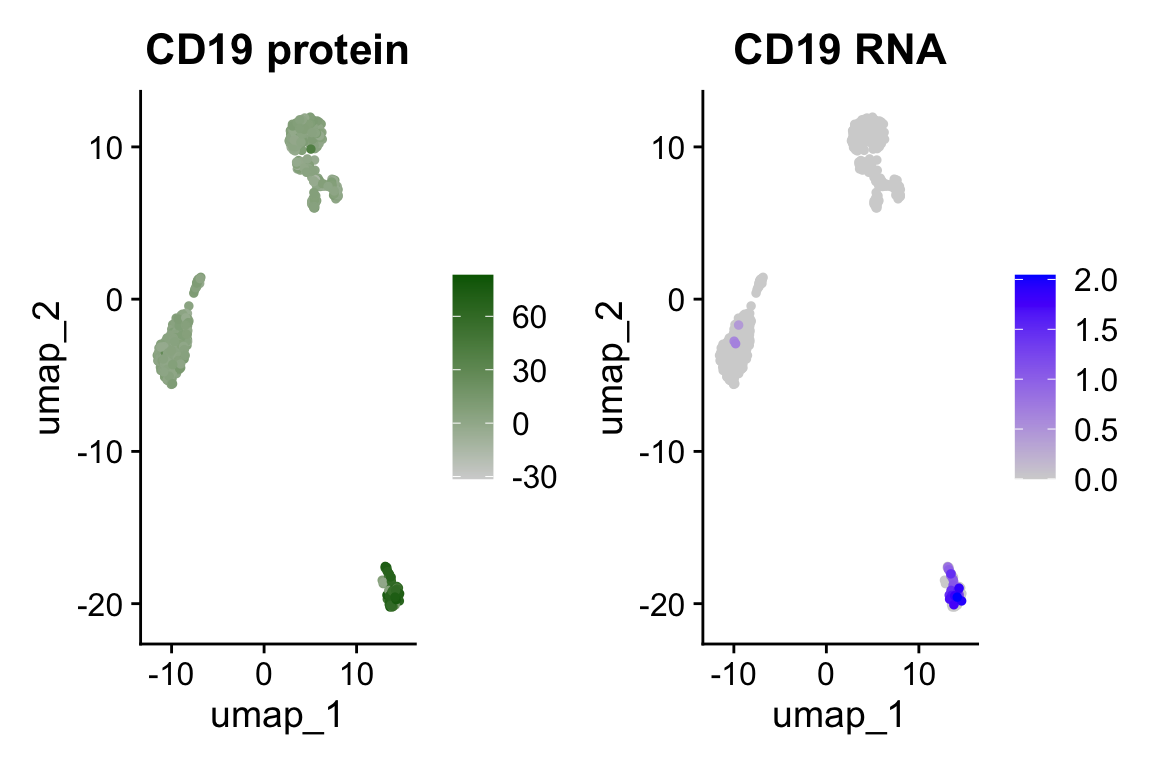
compare it to the Seurat CLR normalization
## clr from seurat
clr_function <- function(x) {
return(log1p(x = x / (exp(x = sum(log1p(x = x[x > 0]), na.rm = TRUE) / length(x = x)))))
}
# use the raw adt counts
clr_norm<- apply(fb_q.filt, MARGIN = 2, FUN= clr_function)Visualize in Ridge plots:
get_expression_meta<- function(obj, mat){
meta<- obj@meta.data %>%
tibble::rownames_to_column(var = "cell")
df<- t(mat) %>%
as.data.frame() %>%
tibble::rownames_to_column(var = "cell") %>%
inner_join(meta)
return(df)
}
# Seurat CLR normalized
p1<- get_expression_meta(pbmc, clr_norm ) %>%
ggplot(aes(x = CD19, y = celltypes)) +
ggridges::geom_density_ridges(aes(fill = celltypes)) +
theme_bw(base_size = 14) +
xlab("") +
ylab("") +
ggtitle("Seurat CLR")
# dsb normalized
p2<- get_expression_meta(pbmc, cells.dsb.norm) %>%
ggplot(aes(x = CD19, y = celltypes)) +
ggridges::geom_density_ridges(aes(fill = celltypes)) +
theme_bw(base_size = 14) +
xlab("") +
ylab("") +
ggtitle("dsb normalized")
p1 + p2 + patchwork::plot_layout(guides = "collect")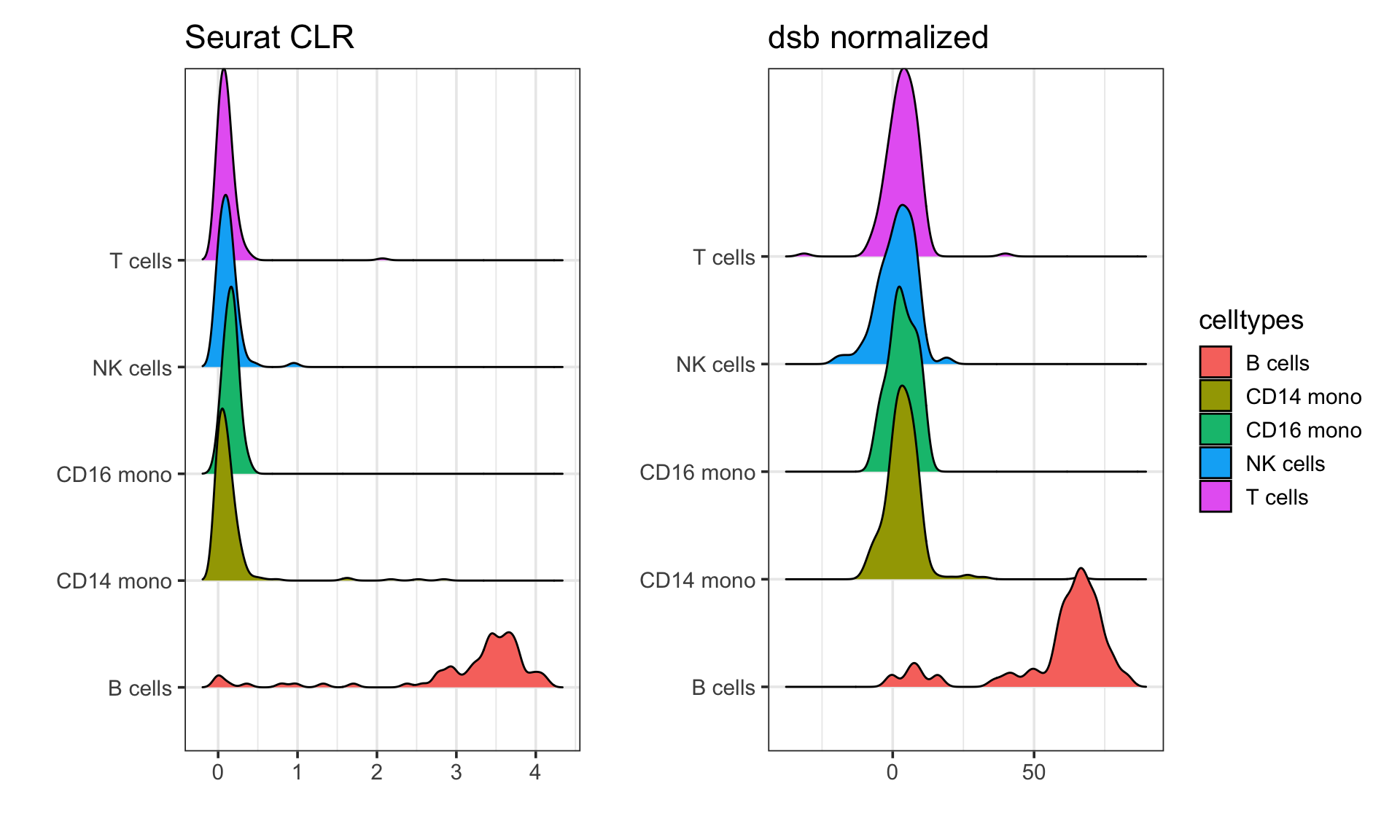
In the paper describing the dsb method, the authors showed that it performs better than the CLR normalization method. You can read the paper for more details.
Happy Learning!
Crazyhottommy